
Just after 07:00 this morning we began recovery of the PAP 1 sensor array. This includes a Met Office ODAS (Ocean Data Acquisition System) buoy.
ODAS measures air pressure, air and sea temperature, humidity, wind speed and direction, wave height and period and was coupled to a mooring wire fitted with marine sensors before being deployed at the PAP site in September 2010. The combination of both the atmospheric sensors and the marine sensors measuring variables such as temperature, chlorophyll (an indicator of productivity) make a powerful tool to understand how climate relates to ocean ecology.
The NMF Moorings Team completed recovery of the buoy that was soon on deck, heavily encrusted in gooseneck barnacles, which needed to be removed. The next task is to add new sensors, batteries, and communication systems to replace those currently on the buoy.
To deploy, operate and recover equipment requires a highly stable platform and we achieve this by operation of the Cook’s dynamic positioning (DP) capability which can render the vessel almost stationary, when ‘on station’. DP is achieved using a suite of tunnel thrusters, an Azimuthing thruster and associated drive units which are controlled, in part, by computer on the Bridge.
Work continued on ODAS throughout the day, with the team working hard to prepare the buoy for its next deployment.

Read more about the ocean observatory.
One of our scientists, Dr Kate Larkin is a biogeochemist who is studying bioluminescence and she writes here about her research...
We are studying the ecology of a group of protists (small single celled organisms) called dinoflagellates and in particular to search for the presence of bioluminescent species here in the Northeast Atlantic. When disturbed, some dinoflagellates give off a blue-white light, visible to the naked eye at night. This fascinating phenomenon is the result of a natural chemical reaction, the oxidation of a light-emitting molecule called luciferin, cataylsed by an enzyme luciferase.
In the dark the result of millions of cells flashing can be spectacular – a carpet of glistening water that can be seen from space. Some areas of the ocean such as coastal waters off Puerto Rico in the tropical Atlantic are famous for their luminescent displays. Here at 49ºN in the Northeast Atlantic, we are not expecting to find such visual displays of luminescence. But bioluminescent species are known to be present here and so we are sampling at different depths in the upper 150m of water to understand what physical and chemical water conditions dictate the vertical profile of dinoflagellate species so we can infer the potential bioluminescent profile in this region of the ocean.
We are measuring bioluminescence using a ‘Glowtracka’. This has been adapted for laboratory use into a bench-top device. The ‘Glowtracka’ is fitted with a photodiode sensor to measure the light flashes and a flow meter to measure the speed that water passes over the photodiode. The photodiode is extremely sensitive, detecting any light emitted at a frequency of 1000 Hz so even the slightest presence of a bioluminescent dinoflagellate should be picked up. From these measurements we can quantify the amount of bioluminescence in a certain amount of water, converting the voltages measured by the photodiode into photons per cm2sec−1.

We are using different methods to study the dinoflagellates at the PAP site. One is conducting incubation experiments to look at how different light levels affect bioluminescence. The other is to look at the vertical distribution of bioluminescent dinoflagellates from the surface to 100m depth. This is done at night-time between 10pm–2am when we expect the highest bioluminescence. We first use a CTD sampler to collect water samples. This is a rosette of 24 bottles, each with a capacity of 10 litres of water. Various sensors are also fitted to the frame to track the profile of temperature, conductivity (to derive salinity) and other variables like chlorophyll-a (a proxy for phytoplankton) so we can look at how these change vertically through the water column. We can choose to open (‘fire’) the bottles at different depths so we collect a profile from the water column.
Once on the surface, the water bottles are emptied into plastic containers that have been blacked out. Because we are sampling at night the dinoflagellates need to be kept in the dark because any light shining on the dinoflagellates may disrupt their natural circadian rhythm as daylight typically inhibits bioluminescence.
In the lab, samples from each depth are passed through the Glowtracka instrument to get a reading of the bioluminescence. In a darkened laboratory the water is poured into a black tube and left to rest for 5 minutes so the dinoflagellate cells can settle. Then a tap is opened so the water flows down through a 2mm mesh gauze which disturbs the cells making the dinoflagellate cells emit visible light.

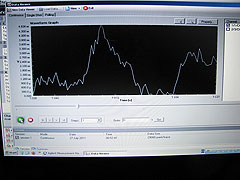
On 26 July we did a CTD cast at ~11pm, taking 80 litres of water samples from 10 depths from the upper 100 m. To decide on water depths to sample we first checked the chlorophyll-a levels in the water using a fluorometer sensor attached to the CTD. This showed a peak in chlorophyll-a (a proxy for phytoplankton) at 8m depth. We focused on investigating what dinoflagellate species were living above, within and below this chlorophyll-a peak and we also sampled down to 100m to see the vertical distribution of dinoflagellates below this chlorophyll-a maximum. Running the samples through the Glowtracka we saw higher voltage levels and therefore Bioluminescence in water from 8m at the peak chlorophyll-a values.

We hope to repeat this sampling tonight to compare with the first profile and to further investigate the vertical distribution of these dinoflagellates in relation to other physical and biological features such as the temperature and salinity gradients and the distribution of other marine phytoplankton. This means another night shift, but on the plus side, we will be awake for another sunrise!
Some facts about dinoflagellates
About 50% of dinoflagellates are primary producers and many have important roles in the marine ecosystem. Perhaps the best known are the photosynthetic dinoflagellates that live inside reef-building corals acting as endosymbiotic zooxanthellae. Others are heterotrophic, grazing on small animals and microbes. Some dinoflagellate species even produce potent chemicals called toxins. In large ‘blooms’ these dinoflagellates can be lethal to marine fauna and can contaminate seafood making it toxic to humans.