A fundamental prerequisite for ocean acidification work is to accurately measure seawater pH and carbon chemistry. The basic objective of ocean acidification research is to work out the impact of inputting extra carbon dioxide into seawater.
We want to establish the effect of these chemical changes on life and other aspects of the oceans. In order to use our experimental and observational results to predict successfully the nature of a future high CO2 world, we have to measure seawater carbon chemistry and pH accurately.
For instance, if we observed a certain group of marine life to die off in one of our experiments at a measured CO2 level of 550ppm (which might conceivably occur before the end of the century) but the CO2 level in the experiment was actually 1500ppm (not likely ever to occur), then the understanding we develop would be highly misleading. Getting the carbon chemistry right is therefore crucial for all of the research carried out on the cruise.

Because it so important, four scientific specialists are involved with this task on this cruise. Today’s blog entry features two of the four: Dorothee Bakker and Gareth Lee from the University of East Anglia at Norwich.
Dorothee and Gareth take alternate long shifts, spending their time in an adapted shipping container (see photo), which sits alongside the bioassay container on the back deck of the Discovery. Although it looks relatively unremarkable from the outside, as you can see from the other photos, the inside is another matter entirely. There are enough pipes, tubes, chemicals and instruments to keep any chemist happy.
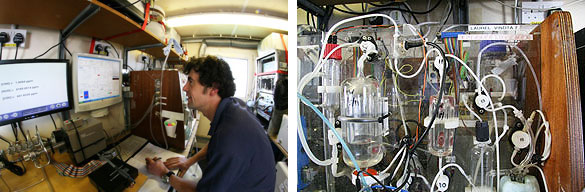
One of the sophisticated instruments in the UEA container measures the ‘partial pressure’ of carbon dioxide (CO2) in seawater, which is also useful for understanding how rapidly it exchanges across the sea-surface. A second instrument, a widely-used workhorse of ocean carbon chemists, is the VINDTA (see photo), which is used to measure both the DIC (the total amount of Dissolved Inorganic Carbon in each litre) and the alkalinity, another property of seawater carbon chemistry. Don’t worry if you are losing the chemical plot here, the main point is that adding CO2 to seawater causes a series of changes which are surprisingly complex to measure and understand – hence the need for good chemists.
As you can see from the photo of the outside of their laboratory, today has been warm and sunny, and indeed several of us have enjoyed sitting out on the back deck. However, the forecast suggests we are heading into rougher weather as we circle round the south of Ireland before heading north into the Irish Sea once more.